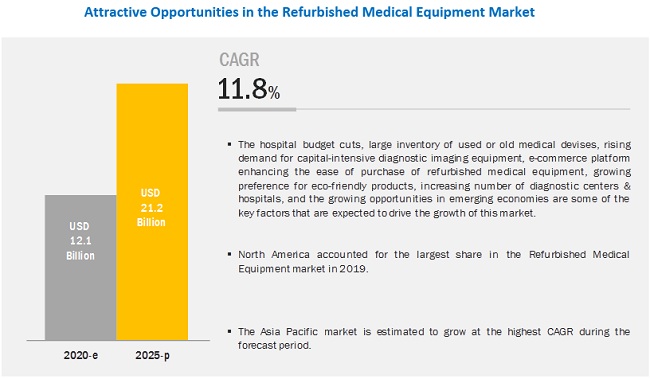
[214 Pages Report] The global refurbished medical equipment market is projected to reach USD 21.2 billion by 2025 from USD 12.1 billion in 2020, growing at a CAGR of 11.8% from 2020 to 2025.
The growth of the global refurbished medical equipment market can be attributed to factors such as hospital budget cuts, a large inventory of used or old medical devises, rising demand for capital-intensive diagnostic imaging equipment, e-commerce platform enhancing the ease of purchase of refurbished medical equipment, growing preference for eco-friendly products, an increasing number of diagnostic centers & hospitals, and the growing opportunities in emerging economies.
However, factors such as stringent regulations on the import and use of refurbished medical devices in certain countries, lack in the standardization of policies for the use & sale of refurbished devices, increase in the influx of low-cost new medical devices, and the negative perception about the quality of refurbished medical devices are expected to restrain the market growth.
Furthermore, the growth of the market is expected to be slowed temporarily due to the COVID-19 pandemic during the forecast period.
Download PDF Brochure: https://www.marketsandmarkets.com/pdfdownloadNew.asp?id=770
Based on product, the refurbished medical equipment market is segmented into medical imaging equipment, operating room & surgical equipment, patient monitors, cardiology equipment, urology equipment, neurology equipment, intensive care equipment, endoscopy equipment, IV therapy systems, and other medical equipment.
The medical imaging equipment segment was estimated to account for the highest share in 2019. The large share of this segment can be attributed to factors such as the presence of a large number of products under this segment and their high utility in the healthcare and clinical space.
Based on procedure, the refurbished medical equipment market is segmented into diagnostic and therapeutic procedures segments. The diagnostic procedures segment accounted for a larger share of the market in 2019.
The rising demand for diagnostic imaging procedures, the high price of new medical imaging equipment, a growing number of target diseases, widening application of diagnostic imaging procedures, regulatory approvals in using refurbished medical equipment, low purchasing power in emerging economies, and established & government validated refurbishment processes adopted by major OEMs are factors expected to drive the growth of refurbished medical equipment market segment in the coming years.
Based on end users, the global market has been segmented into hospitals, ambulatory surgical centers, diagnostic imaging centers, and other end users. The hospitals’ segment accounted for the largest share of the market in 2019.
The rising adoption of refurbished medical equipment, increasing focus of hospitals on providing affordable treatment and care, and growing emphasis on high returns on investment are some of the key factors driving the growth of this market.
Request for Sample Pages: https://www.marketsandmarkets.com/requestsampleNew.asp?id=770
Geographically, this market is classified into North America, Europe, the Asia Pacific, Latin America, and the Middle East and Africa. This can be attributed to the large patient pool in the region, increasing privatization in the healthcare sector, huge patient population base, and high demand for refurbished medical equipment by low-budget hospitals and clinics.
Key Players
GE Healthcare (US), Siemens Healthineers (Germany), Koninklijke Philips N.V. (Netherlands), Block Imaging (US), SOMA TECH INTL. (US), Avante Health Solutions (US), Hilditch Group (UK), Everx Pvt Ltd. (Australia), Integrity Medical Systems, Inc. (US), Radiology Oncology Systems, Inc. (US), Master Medical Equipment (US), US Med-Equip (US), Fair Medical Co. Ltd. (Japan), Future Health Concepts (US), US Medical Systems, LLC. (US), Nationwide Imaging Services (US), Pacific Healthcare Imaging, LLC (US), Venture Medical ReQuip, Inc. (US), Desert Tech Medical Systems (US), Hi Tech International Group, Inc. (US), CURA Healthcare (India), JAPAN CENTRAL MEDICAL, INC. (Japan), HOYU AND CO., LTD. (Japan), Sanrad Medical Systems (India), BOND JAPAN CO., LTD. (Japan), FlexrayMedical ApS (Denmark), Amber Diagnostics (US), Rhombus Medical Equipment., LLC (UAE), Blue Start Limited (India), and Cielo Co., Ltd (Japan).