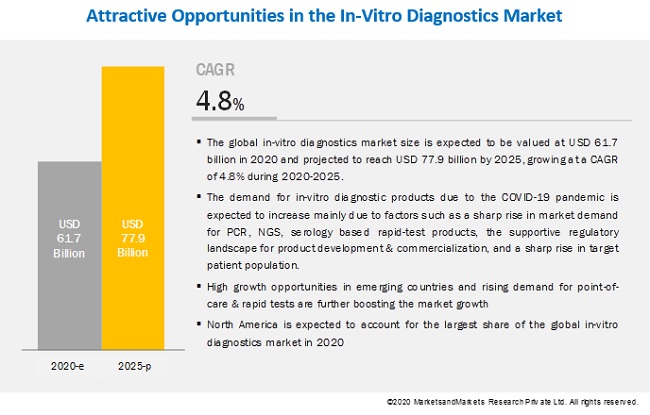
The global in-vitro diagnostics market size is expected to be valued at USD 61.7 billion in 2020 and projected to reach USD 77.9 billion by 2025, growing at a CAGR of 4.8% during 2020-2025. Factors such as the increased global burden of chronic & lifestyle diseases, greater patient emphasis on early and accurate disease diagnosis, increased adoption of automated instruments among end-users, rising demand for point-of-care diagnostic solutions, and higher patient awareness about personalized medicine are expected to drive the market growth of the in-vitro diagnostic products during the coming decade.
Additionally, owing to the unfolding impact of COVID-19 pandemic, the overall in-vitro diagnostics industry is witnessing a sharp rise in market demand for PCR, NGS, serology based rapid-test products, the supportive regulatory landscape for product development & commercialization, and novel strategic initiatives by industry stakeholders to strengthen their manufacturing & distribution capabilities across major healthcare markets.
To know about the assumptions considered for the study download the pdf brochure
New product launches, product development partnerships & collaborations, and geographic expansions are the major growth strategies adopted by leading market players during the last six months to mitigate the impact of COVID-19 pandemic on in-vitro diagnostics market, as well as to capitalize on additional growth opportunities arising due to the pandemic worldwide. Some of the major players that adopted above strategies include Roche Diagnostics (Switzerland), Abbott Laboratories (US), Thermo Fisher Scientific (US), Becton, Dickinson and Company (US), Bio-Rad Laboratories (US), Biomerieux (France), and QIAGEN (Germany), among others.
As of 2019, Roche Diagnostics was the leading player in the IVD market. The company’s leading position in the IVD market is attributed to its diverse product portfolio across clinical chemistry, immunochemistry, molecular diagnostics, and tissue diagnostics products. The company also has a strong geographic presence across major healthcare markets in North America, Europe, Asia, as well as MEA, which enables it to cater to a large consumer base worldwide. To maintain its leadership position in the global IVD market, the company mainly focuses on product commercialization, developmental collaborations, and product distribution partnerships.
Abbott Laboratories accounted for the second-largest share of the IVD market in 2019. The company focuses on strategic acquisitions, geographic expansions, and product development/upgrade as key strategies to withhold its strong position in the global IVD market. For instance, the company had acquired Alere Inc (US) in 2017 that resulted in a significant geographic expansion and capability expansion in PoC diagnostic products segment. Additionally, the company has a strong portfolio of FDA-approved and CE-marked products that have helped them in effective brand positioning across key countries worldwide.
Related Reports:
COVID 19 Impact on IVD (In Vitro Diagnostics) Market by Technology (PCR, NGS, ELISA, Rapid Test, Hematology, Hemostasis, Clinical Chemistry, Microbiology Testing, Urinalysis), End-user and Region – Global Forecast to 2025
Contact:
Mr. Aashish Mehra
MarketsandMarkets™ INC.
630 Dundee Road
Suite 430
Northbrook, IL 60062
USA : 1-888-600-6441
sales@marketsandmarkets.com